The UK started its coronavirus vaccine trial on humans. The first doses of the vaccine have given to the volunteers. Scientists are desperately trying to get a vaccine to fight with coronavirus.
Scientists at the Jenner Institute, University of Oxford, started the first human trial in Europe. The trial injections, which were developed under three months, tried on more than 800 volunteers on Thursday. The first human to be injected for the human trial phase of the vaccine in the UK is a microbiologist.
Trials on 800 Volunteers
Elisa Granato is the first volunteer in a group of 800 which is to be injected first. The results of the vaccine will be the development of immunization against the deadly virus. The positive results of the vaccine will lit a hope of stopping the rapid transmission of the deadly virus.
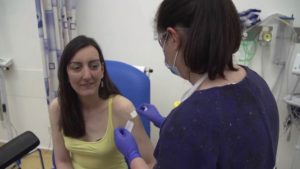
In an interview, Granato told that she is a scientist and she wants to support the scientific process wherever she can. She further said that she doesn’t study viruses and was feeling a bit useless from some days. So she felt like she should support the cause.
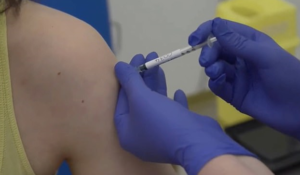
They all will be now monitored for 48 hours to observe the impact of each. Scientists based on the observation will start injecting further volunteers.
“I have a high degree of confidence in this vaccine,” said Sarah Gilbert, professor of vaccinology at the University of Oxford’s Jenner Institute, who is leading the research.
“Of course, we have to test it and get data from humans. We have to demonstrate it works and stops people getting infected with coronavirus before using the vaccine in the wider population,” she said, adding that she remains “very optimistic” about the outcome.
Professor Andrew Pollard, director of the Oxford Vaccine Group, who is leading the trial, said: “We’re chasing the end of this current epidemic wave. If we don’t catch that, we won’t be able to tell whether the vaccine works in the next few months.
“But we do expect that there will be more cases in the future because this virus hasn’t gone away.”
“ChAdOx1 nCoV-19″ vaccine trial
The researchers said that they started the screening of healthy volunteers in March for the “ChAdOx1 nCoV-19″ vaccine trial in the Thames Valley Region of England.
ChAdOx1 nCoV-19 is made from a virus (ChAdOx1), which is a weaker version of a common cold virus (adenovirus) mainly occurring in chimpanzees. They changed it genetically so that it can’t grow in humans.
The main purpose of the trials is to know whether healthy people can be protected from COVID-19. This will also provide them with the safety aspects of the vaccine and the ability to generate good immune responses against coronavirus.
The human trial aims to assess whether healthy people can be protected from Covid-19 with this new vaccine called ChAdOx1 nCoV-19. It will also provide valuable information on the safety aspects of the vaccine and its ability to generate good immune responses against the deadly virus.
The Oxford University team is hoping with ChAdOx1 nCoV-19, to make the body identify and develop an immune response to the spike protein that will help stop the SARS-CoV-2 or Covid-19 virus from entering human cells and therefore prevent infection.
Vaccines made from the ChAdOx1 virus have been given to more than 320 people to date and be safe and well-tolerated. There are temporary side effects, such as temperature, headache or a sore arm.
The patients are provided with the diary to write the experience and all over symptoms occurring seven days from the vaccine. They will do the same for three weeks if they feel unwell.
The volunteers of the vaccine will have to attend the series of follow-ups. In follow-ups, the team of doctors will check observation, blood sample and will review the diary. These blood samples will be used to assess the immune response to the vaccine.
The researchers are in favor of the recruitment of local healthcare workers into the trial. The logic behind this is that they are more likely than others to be exposed to the virus.
Trials in other Countries
A large trial of about 5,000 volunteers will start in the coming months and will have no age limit. The Oxford University team is also doing a vaccine trial in Africa. Possibly in Kenya, where the rates of transmission are growing from a lower base.
The UK government has invested an extra 20 million pound into the University of Oxford trials. They said that it is “throwing everything” at finding a vaccine against coronavirus.





